Hydrogen capacity alludes to the most common way of putting away hydrogen gas in a reduced and proficient way for later use as an energy transporter. As an energy transporter, hydrogen has acquired huge consideration because of its capability to address different energy challenges, especially with regards to sustainable power joining and decarbonization endeavors.
It, most importantly, is a flexible and clean-consuming fuel. At the point when hydrogen is combusted or utilized in energy units, it delivers just water fume as a side-effect, bringing about zero ozone depleting substance discharges. This makes hydrogen a promising option in contrast to petroleum derivatives, which add to environmental change. Besides, hydrogen has a high energy thickness, meaning it contains a lot of energy for every unit mass or volume. Hydrogen is a low-thickness gas at standard temperature and tension, making it trying to store and move productively. We, Nanografi, intently follow your works and undertakings that add to manageability and we unite our items with you for this purpose.
Introduction
The expression “hydrogen capacity” has ignited critical interest in supportable and clean energy arrangements. With its high energy content and potential for zero-outflow applications, hydrogen has arisen as a promising option for a greener future.
From energizing transportation to supporting lattice dependability and empowering environmentally friendly power joining, hydrogen’s adaptability makes it an alluring possibility for tending to the worldwide energy puzzle. In any case, one of the main obstacles to boundless hydrogen reception lies in its efficient stockpiling and transportation. As a lightweight and exceptionally responsive gas, putting away hydrogen in a smaller and safe way has been a longstanding test.

Types of Hydrogen Stockpiling Methods
There are a few strategies for hydrogen capacity, including pressure, liquefaction, and strong state stockpiling.
- Compression: Hydrogen gas can be compacted and put away in high-pressure tanks. This approach considers a significant measure of hydrogen to be put away in a moderately little volume. Notwithstanding, pressure requires energy and can be energy-intensive.
- Liquefaction: Hydrogen can be cooled and changed over into a fluid state, fundamentally decreasing its volume. Fluid hydrogen has a higher energy thickness contrasted with compacted gas. Be that as it may, liquefaction likewise requires a significant measure of energy and cryogenic foundation for capacity and taking care of.
- Solid-state storage: This strategy includes synthetically or truly catching hydrogen inside a strong material, like metal hydrides, substance hydrides, or carbon nanomaterials. Strong state stockpiling offers the potential for high-thickness hydrogen capacity and controlled discharge, yet it actually faces difficulties connected with reversibility, energy, and cost.
Productive and financially savvy hydrogen capacity innovations are urgent for the far and wide reception of hydrogen as an energy transporter. Progresses away techniques are being investigated and created to work on the general proficiency, wellbeing, and reasonableness of hydrogen stockpiling, making it a feasible answer for a scope of uses, including transportation, fixed power, and energy lattice adjusting.

Compressed Hydrogen Storage
Compacted hydrogen capacity is one of the strategies used to store hydrogen gas for different applications. It includes packing hydrogen gas to high tensions and putting away it in uniquely planned tanks or chambers.
Pressure process: Hydrogen gas is compacted utilizing a blower to build its tension. The pressure is regularly finished in various stages to accomplish higher tensions. The most widely recognized pressure levels for hydrogen capacity range from 350 to 700 bar (5,000 to 10,000 pounds for every square inch).
Types of High-pressure Tanks
Storage tanks: Compacted hydrogen is put away in tanks made of high-strength materials fit for enduring the high tensions included. The tanks are intended to guarantee wellbeing, solidness, and honesty during capacity and transportation. Normal tank materials incorporate carbon fiber composites, metal compounds, or a mix of both.
Energy density: Compacted hydrogen capacity offers a somewhat high energy thickness contrasted with other capacity strategies. It considers a lot of hydrogen to be put away in a moderately little volume, empowering applications that require smaller capacity, for example, full cell vehicles.To gain further understanding into this and to fathom how energy units are being changed by forward leaps in nanotechnology, you can peruse our new blog.
Refueling considerations: Compacted hydrogen can be apportioned from stockpiling tanks into energy unit vehicles or other hydrogen-fueled gadgets. Notwithstanding, the refueling system requires specific foundation, including high-pressure apportioning gear and wellbeing measures to deal with the packed gas.
Challenges and Limitations
Compacted hydrogen capacity is broadly utilized in applications like energy component vehicles, where it gives a down to earth answer for putting away and providing hydrogen gas to control the vehicles. In any case, it likewise has a few impediments, for example, the energy expected for pressure, the requirement for high-pressure capacity framework, and the potential for energy misfortune during pressure and decompression processes.
Liquid Hydrogen Storage
Fluid hydrogen stockpiling is a technique for putting away hydrogen in its fluid state, accomplished by cooling hydrogen gas to low temperatures very. Here are the vital parts of fluid hydrogen stockpiling:
Properties of Fluid Hydrogen
Liquefaction process: Hydrogen gas is cooled to temperatures underneath its limit (- 252.87°C or – 423.17°F) utilizing cryogenic refrigeration frameworks. This cycle changes over hydrogen gas into a fluid state, which brings about a huge decrease in volume. The liquefaction interaction requires particular hardware and offices equipped for taking care of cryogenic temperatures.
Storage containers: Fluid hydrogen is put away in protected tanks explicitly intended to keep up with the very low temperatures and forestall heat move from the environmental elements. These tanks are commonly twofold walled with a vacuum between the walls to limit heat entrance.
Energy density: Fluid hydrogen stockpiling offers higher energy thickness contrasted with compacted hydrogen gas. By changing hydrogen from a vaporous to a fluid express, its volume is diminished by a variable of around 1:800.
Handling considerations: Dealing with fluid hydrogen requires severe adherence to somewhere safe conventions because of its super chilly temperature. Specific wellbeing measures, including legitimate defensive hardware, protection, and venting frameworks, are fundamental to forestall wounds and guarantee safe capacity and transportation.
Boil-off and loss: Even with appropriate protection, fluid hydrogen will encounter a bubble off because of intensity entrance. This bubble off should be figured out how to forestall extreme loss of put away hydrogen.
Overview of Metal Hydride Hydrogen
Metal hydrides are intensifies shaped by the blend of metal components with hydrogen. These mixtures show novel properties, especially their capacity to store and delivery hydrogen reversibly. Metal hydrides stand out as potential hydrogen stockpiling materials and track down applications in different fields. Here is an outline of metal hydrides:
Hydrogen capacity: Metal hydrides can store hydrogen through an interaction called hydrogen ingestion. At the point when hydrogen gas comes into contact with a metal hydride, the hydrogen molecules bond with the metal iotas to shape a strong compound. This considers the capacity of hydrogen inside the gem design of the metal hydride.
Reversibility: One of the critical benefits of metal hydrides is their reversibility. They can deliver the put away hydrogen under unambiguous circumstances, like warming or diminishing the strain.
Types of metal hydrides
Metal hydrides can be sorted into two primary gatherings:
Interstitial hydrides: These metal hydrides structure when hydrogen iotas consume the interstitial spaces between metal molecules in the gem cross section. Instances of interstitial hydrides incorporate titanium hydride (TiH2) and vanadium hydride (VHx).
Complex hydrides: These metal hydrides structure when hydrogen responds with metal mixtures or composites to shape more intricate designs. Instances of perplexing hydrides incorporate lithium aluminum hydride (LiAlH4) and sodium borohydride (NaBH4).
A. TIH2: Design AND PROPERTIES
TiH2, otherwise called titanium hydride, is an interstitial metal hydride compound framed by the mix of titanium (Ti) and hydrogen (H). Here is an outline of the design and properties of TiH2:
Structure:
Crystal structure: TiH2 solidifies in the hexagonal gem framework and embraces the space bunch P6/mmm. It frames a layered design with hexagonal close-stuffed (hcp) titanium layers and interstitial hydrogen particles possessing the octahedral destinations between the titanium layers.
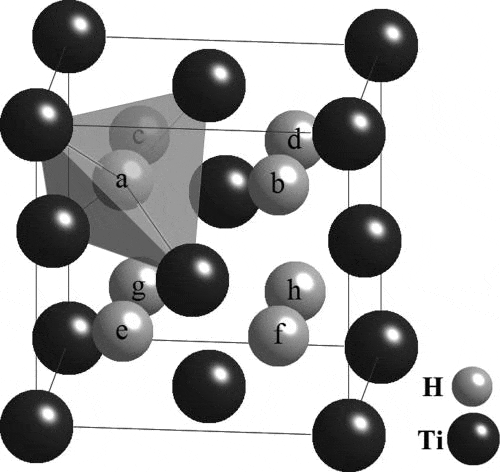
Titanium layers: The titanium particles in TiH2 structure hcp layers, where every titanium iota is encircled by six adjoining titanium molecules. These layers are kept intact by solid metallic bonds.
Interstitial hydrogen: The hydrogen molecules possess the octahedral locales between the titanium layers, shaping TiH6 octahedra.
Properties:
Hydrogen capacity capacity: TiH2 is known for its high hydrogen stockpiling limit. It can retain hydrogen gas and store it inside its gem structure. The specific stockpiling limit relies upon different variables, including temperature, pressure, and the TiH2 organization.
Stability: TiH2 displays great strength under ordinary circumstances. It is steady at room temperature and can hold its hydrogen stockpiling properties overstretched periods.
Reactivity: TiH2 is responsive with water major areas of strength for and, delivering hydrogen gas. This reactivity can be favorable for hydrogen age or as a hydrogen source in unambiguous applications.
Thermal stability: TiH2 has a moderately high warm steadiness, permitting it to endure raised temperatures without huge disintegration. Notwithstanding, over the top warming can make TiH2 discharge hydrogen and go through stage changes.
Density: TiH2 has a somewhat high thickness, with a worth of roughly 3.8 g/cm³. This thickness adds to its capacity to store a lot of hydrogen inside a little volume.
It’s significant that the particular properties of TiH2 can change contingent upon elements like the TiH2 piece, handling strategies, and any doping or adjustments presented during its union. Analysts keep on investigating ways of upgrading the properties of TiH2 and other metal hydrides to improve their hydrogen stockpiling abilities and address any limits they might have for down to earth applications.
B. ZRH2: Construction AND PROPERTIES
ZrH2, otherwise called zirconium hydride, is an interstitial metal hydride compound shaped by the mix of zirconium (Zr) and hydrogen (H). Here is an outline of the construction and properties of ZrH2.
Structure:
Crystal structure: ZrH2 takes shape in the hexagonal gem framework and embraces the space bunch P6/mmm, like TiH2.
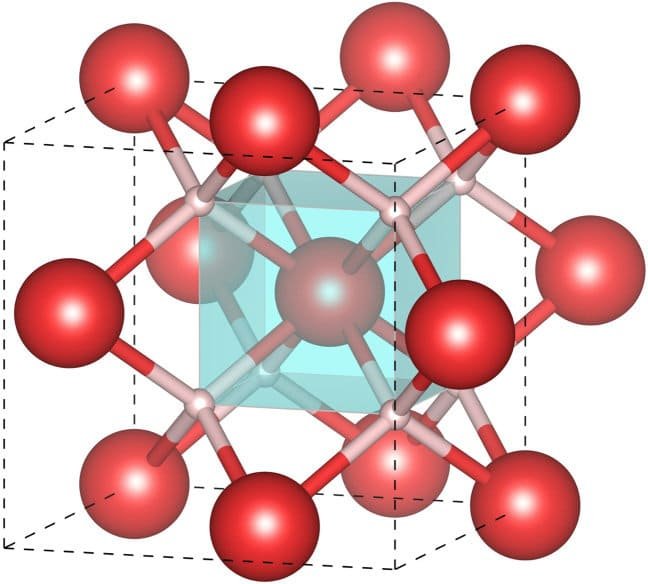
Zirconium layers: The zirconium iotas in ZrH2 structure hcp layers, like TiH2. Every zirconium iota is encircled by six adjoining zirconium particles, making solid metallic bonds.
Interstitial hydrogen: The hydrogen molecules involve the octahedral locales between the zirconium layers, shaping ZrH6 octahedra. Every hydrogen molecule is attached to three adjoining zirconium iotas. The presence of interstitial hydrogen gives ZrH2 its hydrogen stockpiling limit.
Properties:
Hydrogen capacity capacity: ZrH2 shows a prominent hydrogen stockpiling limit. It can retain hydrogen gas and store it inside its precious stone design. The capacity limit relies upon different elements, like temperature, pressure, and the organization of ZrH2.
Stability: ZrH2 is by and large stable under ordinary circumstances. It holds its hydrogen stockpiling properties overstretched periods at room temperature. In any case, as other metal hydrides, ZrH2 can go through primary changes and stage changes under specific circumstances, influencing its hydrogen stockpiling execution.
Reactivity: ZrH2 can respond with water areas of strength for or, delivering hydrogen gas. This reactivity can be profitable for hydrogen age or as a hydrogen source in unambiguous applications.
Thermal stability: ZrH2 has great warm strength, permitting it to endure raised temperatures without critical decay. Notwithstanding, unnecessary warming can make ZrH2 discharge hydrogen and go through stage changes.
Mechanical properties: ZrH2 shows great mechanical properties, including high strength and hardness. These properties make it valuable in applications where both hydrogen stockpiling and mechanical strength are required.
It’s essential to take note of that the particular properties of ZrH2 can differ contingent upon elements like the ZrH2 creation, handling strategies, and any doping or alterations presented during its combination.
Advantages and challenges:
Metal hydrides offer a few benefits as hydrogen stockpiling materials. They can accomplish high hydrogen stockpiling limits, have great dependability, and are for the most part protected to deal with. In any case, challenges remain, including the requirement for upgraded response energy for effective hydrogen take-up and deliver, the advancement of lightweight and minimal expense materials, and addressing thermodynamic impediments to improve capacity limits.
Applications:
Metal hydrides have applications past hydrogen stockpiling. They are utilized in hydrogen purging and partition processes, hydrogen power devices, nuclear power stockpiling, heat siphons, and hydrogen sensors. Metal hydrides likewise have possible in different fields, like hydrogenation responses in compound cycles and as impetuses in different modern applications. Innovative work endeavors are centered around investigating new metal hydrides, upgrading their properties, and working on their exhibition for hydrogen capacity and different applications.
Chemical Hydride Storage
How Compound Hydrides Work
Substance hydrides are a class of mixtures that can store and delivery hydrogen through synthetic responses. Dissimilar to actual capacity techniques like compacted or condensed hydrogen, synthetic hydrides depend on substance responses to tie and delivery hydrogen reversibly. Here is an outline of how synthetic hydrides work:
1. Hydrogen assimilation
2. Substance holding
3. Hydrogen discharge
4. Energy input
5. Reversibility
6. Hydrogen capacity limit
Compound hydrides offer a few benefits, including high hydrogen stockpiling limits, the capacity to store hydrogen at encompassing temperatures and tensions, and the potential for controlled and on-request hydrogen discharge. Nonetheless, they likewise face difficulties like response energy, thermodynamics, and the requirement for reasonable impetuses or conditions to work with hydrogen discharge. Innovative work endeavors center around finding new compound hydrides, advancing their properties, and investigating strategies to improve the proficiency and reasonableness of substance hydride-based hydrogen capacity frameworks.
Different Sorts of Compound Hydrides
There are different sorts of compound hydrides that can store and delivery hydrogen through synthetic responses. These sorts can be classified in light of the idea of the substance holding and the components in question. Here are a few normal kinds of substance hydrides:
1. Ionic hydrides
2. Covalent hydrides
3. Complex metal hydrides
4. Amides and imides
5. Borohydrides
6. Natural hydrides
Challanges and Advantages
These are only a couple of instances of the various sorts of synthetic hydrides. Each type has its own qualities, hydrogen capacity limit, and conditions for hydrogen discharge. Scientists proceed to investigate and foster new synthetic hydrides with further developed properties, for example, upgraded hydrogen capacity limits, lower working temperatures, and more proficient hydrogen discharge components.
Carbon-based Materials for Hydrogen Storage
Carbon-based materials stand out as possible contender for hydrogen capacity because of their extraordinary properties and adaptability. Here are a few instances of carbon-based materials utilized for hydrogen capacity:
- Carbon nanotubes (CNTs)
- Graphene
Carbon nanofibers (CNFs) - Porous carbon materials
- Metal-natural structures (MOFs)
- Carbon-based hydrogen capacity composites
Carbon-based materials for hydrogen capacity offer benefits like minimal expense, overflow, and tunable properties. Nonetheless, challenges remain, including improving the hydrogen stockpiling limits, upgrading the energy of hydrogen take-up and deliver, and tending to the dependability and reversibility of the hydrogen stockpiling process.
Borophene: A Promising Carbon-based Material for Hydrogen Storage
Borophene is a two-layered (2D) allotrope of boron that shows an extraordinary nuclear design and interesting properties. Here is an outline of the construction and properties of borophene:
Structure
Atomic arrangement: Borophene comprises of a solitary layer of boron iotas organized in a hexagonal cross section. The boron iotas structure three-sided or hexagonal units, making a honeycomb-like construction. The game plan of boron molecules can change, prompting different borophene structures with particular properties.
Defects and patterns: Borophene can display different imperfections, for example, opportunities or replacements, which can essentially impact its properties. Also, borophene can shape various examples, including stripe-like, permeable, or holey designs, contingent upon the plan of boron molecules.

Properties:
Metallic nature: Borophene is commonly viewed as a metallic material, meaning it conducts power well. The metallic way of behaving emerges from the to some extent filled electronic groups in the borophene structure, which consider the free development of electrons.
Anisotropic properties: Borophene displays anisotropic properties, significance its properties contrast along various headings inside the material.
Mechanical strength: Borophene is known for its extraordinary mechanical strength. It can endure huge strains without breaking because of solid covalent holding between boron molecules.
Thermal conductivity: Borophene displays high warm conductivity, empowering proficient intensity move.
Chemical reactivity: Borophene is artificially responsive and can interface with different substances.
Potential for hydrogen storage: Its 2D design and the capacity to adsorb hydrogen particles on its surface make it a promising contender for hydrogen capacity applications.
It’s vital to take note of that borophene is a generally new material, and examination on its construction and properties is as yet progressing. Researchers are investigating different amalgamation techniques and exploring the likely uses of borophene in different fields, including hardware, energy capacity, catalysis, and that’s just the beginning. The one of a kind design and properties of borophene make it an interesting material for additional investigation and likely innovative progressions.
Carbon Nanotubes and Graphene (Graphene and CNT)
Carbon nanotubes (CNTs) and graphene are both carbon-based materials with interesting designs and remarkable properties. Here is an examination among graphene and carbon nanotubes:
Graphene
Structure: Graphene is a solitary layer of carbon iotas organized in a hexagonal cross section. It shapes a two-layered (2D) honeycomb structure.
Electrical conductivity: Graphene is a fantastic director of power because of its novel electronic band structure. It takes into account the free development of electrons, making it exceptionally conductive.
Mechanical strength: Graphene is major areas of strength for staggeringly has a high elasticity. It is one of the most grounded materials known, with remarkable mechanical properties.
Optical properties: It is straightforward and retains just a little part of light across an expansive scope of frequencies, making it a promising material for optoelectronic applications.
Thermal conductivity: Graphene has extraordinarily high warm conductivity, permitting it to move heat proficiently. This property makes it alluring for warm administration applications.
Flexibility: Graphene is profoundly adaptable and can be twisted, collapsed, or extended without breaking. This adaptability makes it reasonable for adaptable hardware and different applications that require bendable materials.
Explore the 60 application areas of graphene, the most broadly perused subject on our blog, by visiting our website.
Carbon Nanotubes (CNT)
Structure: CNTs are tube shaped structures made out of rolled-up graphene sheets. They can have various designs relying upon the course of action of the carbon molecules, including single-walled (SWCNTs) or multi-walled (MWCNTs) nanotubes.
Electrical conductivity: CNTs display brilliant electrical conductivity, like graphene. They can go about as metallic or semiconducting materials, contingent upon their design and width.
Mechanical strength: CNTs are areas of strength for uncommonly have high elasticity. They can endure huge mechanical anxieties and strains without breaking.
Aspect ratio: CNTs have a high viewpoint proportion, meaning their length is fundamentally more noteworthy than their breadth. This exceptional viewpoint proportion makes them valuable in applications requiring support or as building blocks for nanoscale gadgets.
Applications: CNTs have tracked down applications in different fields, including hardware, energy capacity, composites, sensors, and nanomedicine, because of their uncommon properties and nanoscale aspects.
While both graphene and CNTs share a few properties, like high electrical conductivity, mechanical strength, and warm conductivity, they contrast in their designs and applications. Graphene’s 2D design and extraordinary electrical and optical properties have prompted its utilization in gadgets, photonics, and energy-related applications. Then again, CNTs’ extraordinary rounded design and high perspective proportion make them reasonable for support, nanoelectronics, and other nanoscale applications. The two materials keep on being broadly contemplated and hold incredible commitment for future innovative headways.
Porous Materials and Metal-natural Structures (MOFs)
Permeable materials, like metal-natural systems (MOFs), are described by their capacity to consolidate and store visitor particles inside their permeable design. They offer high surface regions and custom fitted pore structures, making them appropriate for different applications. MOFs, specifically, are made out of metal particles or groups associated by natural ligands, shaping a glasslike structure with consistently dispersed pores. They have tunable properties and have shown guarantee in gas capacity and partition. Notwithstanding, difficulties, for example, security should be tended to. Generally speaking, permeable materials, including MOFs, keep on being a functioning area of examination with expected applications in energy, climate, and different fields.
Hydrogen Sorption Mechanisms
Hydrogen sorption components include the adsorption or retention of hydrogen gas onto a material for stockpiling purposes. The primary systems incorporate physisorption and chemisorption, as well as a mix of both.
Physisorption is the actual adsorption of hydrogen onto a material’s surface, fundamentally through powerless van der Waals powers. It is seen in materials with an enormous surface region, like enacted carbon, metal-natural structures (MOFs), and certain zeolites.
Chemisorption includes a substance response among hydrogen and the stockpiling material, framing compound bonds. It normally requires higher temperatures and in some cases the presence of an impetus. Chemisorption happens through processes like dissociative chemisorption, where hydrogen particles separate into individual molecules that security with the material. Instances of materials that show chemisorption incorporate metal hydrides, complex metal composites, and certain MOFs.
A few materials can show a blend of physisorption and chemisorption systems. Metal-natural systems, for example, may have actual adsorption destinations as well as metal habitats for synthetic holding. This mix takes into consideration upgraded capacity limits and further developed energy.
The decision of hydrogen sorption component relies upon variables like capacity limit, working circumstances, and explicit application prerequisites. Progressing research intends to foster new materials and systems for further developed hydrogen capacity, helping applications like energy units and hydrogen-controlled vehicles.
Future Patterns and Challenges
Future patterns and difficulties in hydrogen sorption components and capacity innovations rotate around creating progressed materials with higher capacity limits, further developing thermodynamics and energy, laying out a vigorous hydrogen framework, tending to somewhere safe contemplations, decreasing expenses, coordinating with sustainable power frameworks, laying out principles and guidelines, and assessing ecological effects. Conquering these difficulties will be fundamental for the broad reception of hydrogen as a spotless and effective energy transporter.
Technological Advancements
Mechanical progressions in hydrogen sorption components and capacity have prompted the improvement of cutting edge materials, for example, metal-natural structures and carbon nanomaterials, with higher capacity limits and further developed energy. Nanotechnology and impetuses play played critical parts in upgrading hydrogen adsorption properties and response rates. Complex hydrides and half and half stockpiling frameworks joining various systems have been investigated for upgraded execution. High level portrayal procedures and computational displaying have given important experiences to material plan and framework improvement. These headways add to making hydrogen stockpiling more effective and commonsense for different applications.
Conclusion
Hydrogen capacity techniques incorporate compacted gas, fluid hydrogen, metal hydrides, synthetic hydrides, and carbon-based materials. Every technique enjoys its benefits and difficulties, and the decision relies upon variables like capacity limit, security, and cost. Progressing research plans to foster high level materials and frameworks for productive hydrogen stockpiling. Hydrogen has solid potential as a perfect energy arrangement because of its flexibility, capacity to store sustainable power, decarbonization benefits, high energy thickness, various applications, mechanical progressions, and global help. Challenges remain, yet hydrogen is ready to assume an essential part in the progress to a perfect and maintainable energy framework. We are devoted to creating and delivering items that line up with our central goal for a cleaner and more feasible energy future.



